- Version
- Download 21
- File Size 4.00 KB
- File Count 1
- Create Date 2023-04-06
- Last Updated 2025-03-17
Freq Wavelengths
These slides are all about the Speed of Light. We all know about the speed of light, right? …Oui?…Si? ("…no, just plain 'c', jokes the pretend engineer").
[To just download the slides, click the blue button to the left, put in the passcode QC_b4_QA.]
A fun trick is to take the Frequency of the color Orange in hertz (or Hz or actually …what is that science number for trillion? Terahertz – meaning 12 zeros)) and multiply that frequency by the Wavelength of Orange (in meters, though so small that it is a zero, a decimal point then nine zeros – the science number is that hip number 'nano' – the total is, 500 Terahertz multiplied by 600 nanometers, which equals 300,000,000 meters per second.
Another example is Blue. Multiply the Frequency by the Wavelength: 666 x 1012 x 450 x 10-9 = 3.00 x 108 meters/second (that is 3 with 8 zeros, or again, 300,000,000 meters per second.)
299,792,458 is the exactly correct number for "Speed of Light", but we can use approximate numbers since …why not? …it is memorable. …and since they (the different frequencies) all change slightly in different circumstances. Some of the variables include defining exactly "What is Blue?" and what material is the light going through? …the vacuums of empty space or a glass prism or water? ...so 300 thousand kilometers a second is good number to use for the Speed of Light …and memorable. (A kilometer is, of course, 1,000 meters, so 300,000,000 meters equals 300,000 kilometers. So, yes, the 'c' in Einstein's famous formula for Energy (E=mc2, that is, the Energy of an object in motion is the mass of the object times the square of the speed of light. We can look into what mass and energy are in another discussion.)
Sometimes you will see the chart going one way, with the size of the wave ascending (the wavelength getting larger – like this one does) and sometimes you will see it with the frequency of the wave ascending shown increasing from left to right. In this case, the the speed the wave – the frequency or cycles per second – are fastest on the left, and slower on the right, which also translates to more energy on the left and less on the right). No matter which direction, frequency and wavelength will correspond to each other in the inverse – one set of numbers increasing and one set of numbers decreasing – and always tied to the speed of light. The science (or math) term for this type of relationship is "inversely proportional". (Bonus points for anyone who can work that into a love song to their significant other.)
As a sidenote before the technical specs of the slides, the frequency of the tuning note on a piano – the "A" note above middle "C" – is 440 Hz. The word "Hertz" isn't used because it comes from some ancient civilization that studied light or sound or ripples in water. Hertz is the family named for a clever scientist who figured out a lot of different things.
In his honor the word "Hertz" was chosen to represent "Cycles per Second". The abbreviation is Hz. A thousand cycles per second is noted as kHz. …or 1 kHz, with a space between the digit and the term.
A frequency does not have to be "per second". For example, the frequency that we travel in a car is kilometers per hour.
In simple terms, sound is a wave and a pure tone is a pure wave. If a wave is going through water (so you can see it) instead of air (which you cannot see), and you measure the top of the peak of one wave to the top of the peak of the next wave, that measurement is the Wavelength.
If you count how many of those peaks go by a certain mark every second, that is the Frequency. A simple example is to look at or lightly touch the strings of a piano or guitar as they vibrate back and forth. On the low notes you can see the motion, or feel the motion distinctly with your finger.
Back to our "A" above middle "C" – which is too fast to see, actually – it is interesting to compare those musical waves with the waves in light. Are the number of cycles per second of a particular frequency of light a thousand times more than the cycles per second of the note on the piano? …a million? …a billion?
if we multiply this 440 cycles per second – by
[ ] 1,000 times – that is, a thousand times – that number is too small
[ ] 1,000,000 times – that is, a million times – still too small of a number
[X] 1,000,000,000 times – that is, a billion times – yes, the correct amount~!
[ ] 1,000,000,000,000 times – that is, a trillion times – OK; a bit too much
…the total of that multiplication would be the frequency of red light as it transitions to infrared light. 440 x a billion cycles per second. Or, to avoid counting the zeros, the science people say 440 times 1012. (440 times 10 to the 12th power – or for my simple mind, 440 followed by 12 zeros.)
The sensors called our 'eyes' almost can not see it. The sensors of our skin might sense it as heat. But the point is that the frequency of the middle notes of the piano are a billion times lower than the frequency of red light.
By the way; the Frequency and the Wavelength of Sound are also tied to each other – but it is different. There are more things to consider. Light doesn't care much if it travels through the vacuum of space or glass or water …a little bit of change, but not much – and when it gets in the clear again, it goes back to its original speed. With sound, it really matters what it is going through. It is just as fascinating. I remember my first lesson; with low frequencies, all you get to affect it with is mass and distance. And that is what makes it a great topic for another time.
The TIFF files here are 4K, 4096 (not 3840) x 2160, 16 bit RGB in the 2020 color space.
This zip file is composed of 4 slides;
- the lower layer with the colors in their full monochromatic variation (without white or black added, though it should be noted that since the slide has an alpha channel that allows the background to leak through, that there is also a white layer below them – figuring that this would be shown on a white screen anyway. [in fact, in this version the background is black],
- one slide of a gradient black on the bottom to white on the top which, when laid over in Hard Light mode (not Screen) creates the variation of the color space (presuming that the white point is declared, which is D65),
- one with the numbers and
- one with the title.
- UPDATE: Each slide now has a twin with a light grid overlay to assist in the Just Noticeable Difference Quest.
Hope you learn or teach and have as much fun using this as I had making it.
About those little numbers on the center line…
The passcode is QC_b4_QA
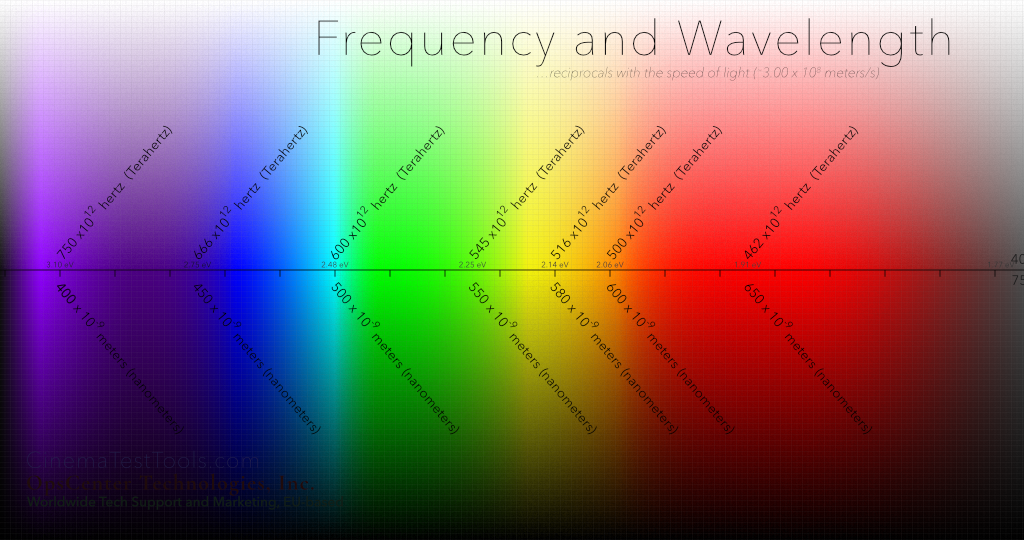
About those little numbers on the center line…
There is one more story on this graphic, which tells of the amount of energy that goes flying off when the electron associated with the photon changes its orbit. This energy is greater on the left side, where the wavelength is shorter and the cycles per second are greater – you can just imagine that the potential amount of energy increases, similar to when the revs of your car increase. Let's talk about that.
The car is measured in revolutions per minute, or RPM. …usually thousands of RPM. Usually, this RPM increases as the speed of the car increases – at least, that is what it seems like. Actually, the driver increases the RPM, getting more energy from the engine – and the speed of the car increases. The potential of the car's speed is in the energy source – the gas and the explosion inside the engine that converts the energy in the gas (or battery) into the motion of the wheels.
Light has many properties and making electrons appear is part of their magic. It is actually a conversion, like heat from an oven turning into bread – if the other ingredients are in the right place and time as well.
In any case, this power in the light that converts to electrons is measured in eV – for electron Volt – which is a unit of energy that indicates the potential of the conversion to do things. The shortest wavelength in the visible spectrum is, of course, in the violet side. We know that as the waves get shorter and go invisible to the eyes of humans, they slip into the area called x-rays and gamma rays and others that we now know are actually very destructive to human tissue. But this is all, yet again, a different story – like tangents in rain.
Energy in eV
|
Violet (limit) |
3.10 |
| Blue |
2.75 |
|
Cyan |
2.48 |
|
Green |
2.25 |
|
Yellow |
2.14 |
|
Orange |
2.06 |
|
Red |
1.91 |
| Red (limit) | 1.77 |
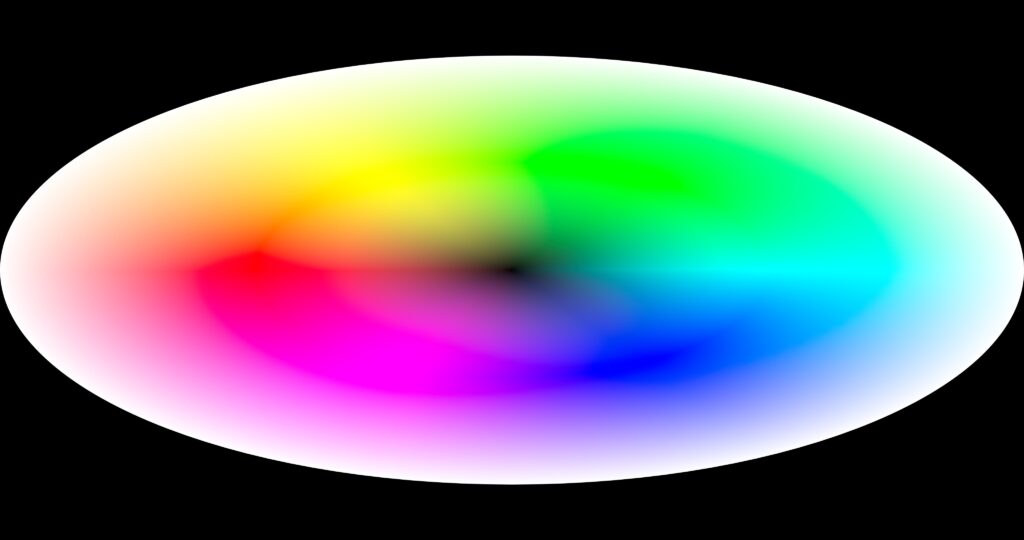
The following experiments in the attempt to put the entire color space onto a flat display spins the colors in a radius instead of a linear line. Since it is designed to fit a movie screen, it is wider than high. …and was a lot of fun to make. The idea includes an optional overlay that gives the eye a bit of a clue on how to see Just Noticeable Differences as the shades of where you are looking go toward another color. There are also versions with white in the center going out to black.
For a complete look at all of our color slides for cinema, see:
